Copper is a very active metal, easily making changes when touching the environment. This activity leads to changes on the coin’s surface.
Patina is a natural change in the coin’s surface color, happening very slowly over a long time. This stable chemical process creates a protective layer on the copper surface, this patina being a desirable sign for coin collectors and a chance to see a high initial price in the Coin ID Scanner app.
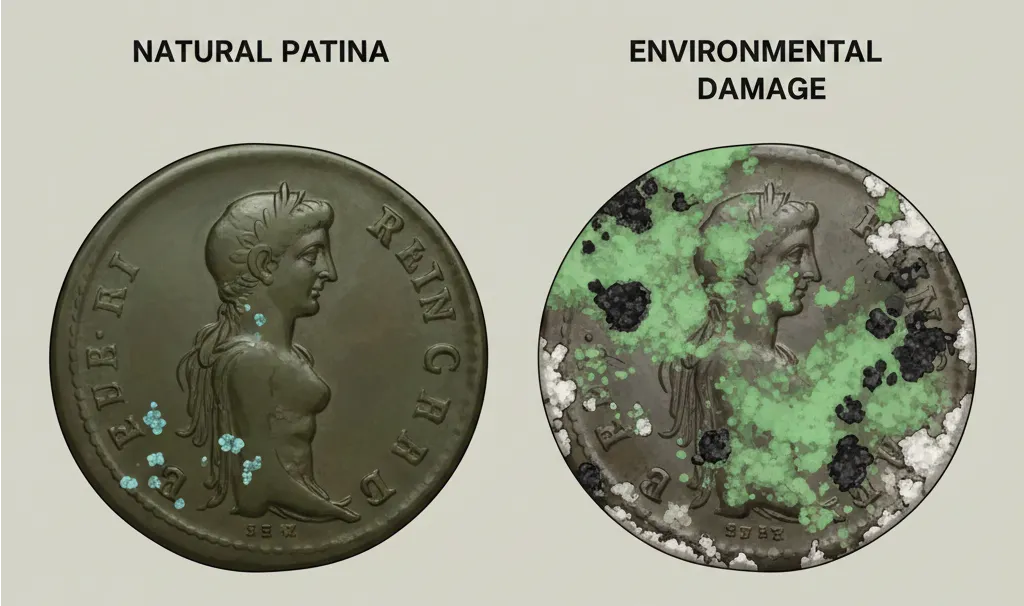
Environmental Damage is a fast, aggressive, or uncontrolled chemical action on the coin’s surface. This action causes corrosion, creating unwanted spots or residue, this environmental damage actively destroying the coin’s surface and always lowering the coin’s value.
How Patina Forms
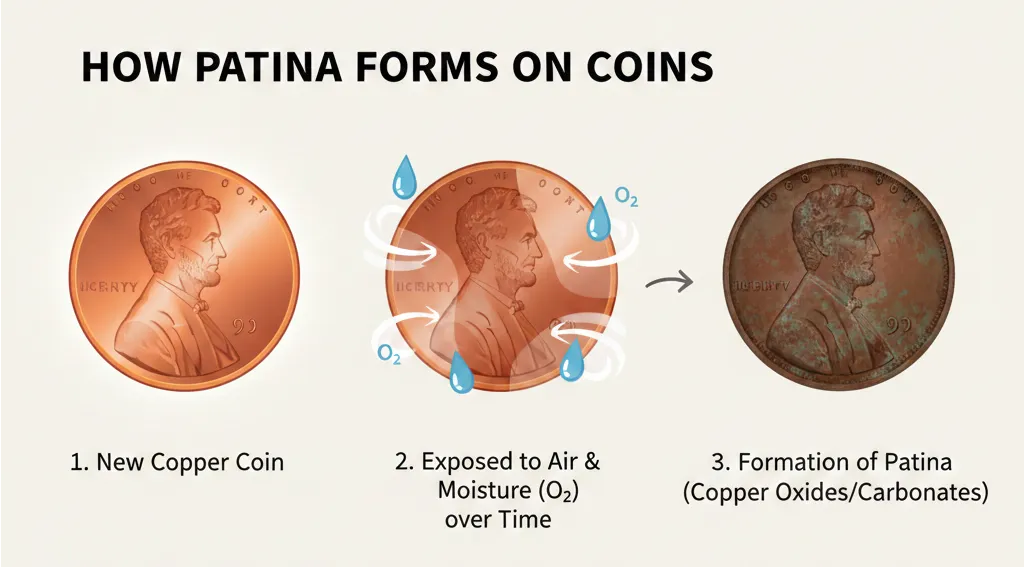
Patina forms when copper reacts with a very small amount of oxygen and sulfur in the air, this chemical change being called oxidation. The process is very slow and gradual, needing many years to complete.
- Patina is a very thin, hard, and stable layer of copper oxides or copper sulfides. This layer sticks tightly to the coin’s metal, never crumbling or falling off the coin.
- After forming, the patina acts as a protective barrier. It slows down copper’s future contact with air and moisture, this patina therefore protecting the pure metal of the coin from more serious destruction that could happen later.
- Good patina on copper coins usually shows shades of brown, dark red, chocolate, or black color. These colors are signs of long, stable, and safe storage.
Some coins, kept in special places, like in paper albums, can show rainbow toning with soft blue or green colors, these blue or green colors also being good only if they are very even, clear, and stable, showing no powdery or chalky deposits.
- Patina always covers large areas of the coin surface evenly. It makes the coin’s details look sharper, never hiding the small features. A good patina shows that the coin is real and has a long history.
Reasons Patina Is Valuable
Patina increases a coin’s value for several important reasons, these reasons being directly related to the coin’s condition and history.
The presence of a stable, old patina confirms that the coin was not cleaned aggressively or treated with strong chemicals. This proves the coin has kept its original condition, collectors valuing this originality very highly.
The deep, rich shades of patina, for example, a strong chocolate brown or dark red color, are thought to be beautiful. A coin having a good patina looks better and more noble than a coin without any color or a coin cleaned until it shines.
- As mentioned before, patina is a stable form of oxidation, stopping more destructive corrosion from starting. Collectors value this protective layer, it guarantees the coin’s future preservation and safety.
Environmental Damage
Environmental damage happens because of aggressive and fast exposure to moisture and bad chemicals. This exposure does not create a stable protective layer, but actively destroys the metal instead.
- The most common reason for destruction is long exposure to water or aggressive salts, these salts being found in soil, in bad storage containers, or in very wet air.
- Verdigris is the most dangerous form of corrosion for copper. Verdigris appears as powdery, often bright green or blue-green spots, these spots being copper compounds like carbonates or chlorides.
- Properties: Verdigris is active, continuing corrosion. It looks like fuzzy or crystalline patches, these patches being easily removed by scratching.
- Destruction: Verdigris goes deep into the metal, creating small holes and craters. Even after removing the verdigris, damage remains on the coin’s surface, this damage being permanent and irreversible.
- If a coin is stored in old, soft plastic holders, these holders being made of polyvinyl chloride (PVC), the plastic releases chemicals over time. These chemicals react with the copper, causing damage.
- Properties: PVC residue looks like a sticky, green, clear, or light grey film. This film also aggressively eats away the copper, creating spots, this deposit not being a stable patina.
Contact with acids, for example, from fingerprints, sweat, or factory dirt, also causes aggressive surface etching, creating uneven, dull, or too-bright spots.
| Sign | Desirable Patina | Destructive Deposits |
| Color | Even, deep, rich (brown, dark red, black) | Uneven, bright, unnatural (bright green, blue-green, white, sticky grey) |
| Texture | Smooth, dense, the coin feeling solid and flat | Powdery, crystallized, crumbling, sticky, or layered |
| Stability | Cannot be removed without strong chemical or mechanical cleaning | Can be removed, but leaving pits underneath |
| Effect on Details | Makes the coin’s relief details clearer | Leads to holes and roughness on the surface |
| Spread | Covers the whole coin or large, clear areas | Local, random spots, often starting in recessed areas or near the edges |
Effect on Collector’s Value
The condition of the surface is a very important factor deciding the price of any coin by the best coin identifier app, especially copper ones.
- Perfect Patina: A coin having an even, attractive, deep brown or red patina will get a high grade. Such a coin sells for a higher price than the same coin after being cleaned, the patina therefore adding value.
- Verdigris and PVC Residue: Any sign of active corrosion, verdigris, or PVC residue immediately lowers the coin value, often very much. A coin having verdigris cannot get a high grade, collectors not wanting to buy a coin that is still destroying itself.
Professional grading companies always clearly note the presence of corrosion. They might give a coin with aggressive deposits a “Details” grade instead of a number grade, this meaning the coin has surface problems and its value is much reduced. A perfect patina, on the other hand, helps the coin get a high number grade.
Storage and Preventing Damage
Only use special, safe containers for coins. They must be made of inert plastic, like Mylar or polystyrene. Never store coins in soft plastic albums or holders, those possibly containing PVC. PVC releases aggressive chemicals, causing the sticky green residue.
Don’t touch the coin surface directly as fats and salts on your fingers can create spots, these spots turning into corrosion over time. Always hold the coin only by its edge.
Don’t try to remove patina or spots from a copper coin yourself because mechanical cleaning or using strong chemicals almost always damages the coin surface forever, lowering its collector’s value. Removing patina or corrosion should only be done by experts.